ANSWER
The mass of the sample is 52.69 grams
Step-by-step explanation
Given data
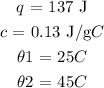
To find the mass of the sample, we will need to apply the below formula
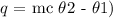
The next step is to substitute the above data into the above formula

Hence, the mass of the sample is 52.69 grams