Answer:
![\mleft[H_3O^+\mright]=0.025M](https://img.qammunity.org/2023/formulas/chemistry/college/ltn7x6uxwlxnmb29up5tbf73nhzdghu58y.png)
Step-by-step explanation:
Here, we want to get the solution with the highest pH
What is pH?
pH is the negative logarithm to base 10 of the concentration of the oxonum ion
Mathematically, we have this as:
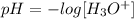
By this, we can get the pH value
We have different oxonium ion concentrations
What we have to do here is to find the relationship between pH and the concentration of the oxonium ions
It is known that, the lower the pH value, the greater the oxonium ion value
So, there is an inverse relationship between pH values and the concentration of the oxonium ion
Thus, the solution with the highest pH value will be the solution with the lowest concentration of oxonium ion
The correct answer here is 0.025 M