Answer;
4.60moles
Explanations:
The number of moles and volume are related to each other by the formula;
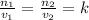
where
n1 and n2 are the initial and final number of moles respectively
v1 and v2 are the initial and final volumes respectively
Given the following parameters
• n1 = 3.00moles
,
• v1 = 550mL = 0.55L
,
• v2 = 845mL = 0.845L
Required
Final mole "n2"
Substitute the given parameters into the formula
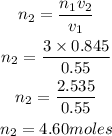
Hence the number of moles will increase to 4.60moles