Answer:
The volume of the ballon is 4.48L.
Step-by-step explanation:
1st) It is necessary to make sure that all the variables units are in atm, L, mol and K. So, we have to convert 0.00°C to K:
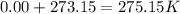
2nd) With the ideal gas formula, we can replace the values of pressure, number of moles and temperature, to calculate the volume of the helium gas in the ballon:
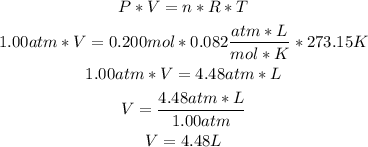
R in the formula is the ideal gas constant, and it is equal to 0.082 atm*L/mol*K.
So, the volume of the ballon is 4.48L.