Answer:
The pH of the solution is 11.7 (12).
Step-by-step explanation:
1st) The reaction between KOH and HBr is:

According to the balanced reaction, 1 mole of KOH neutralize 1 mole of HBr.
2nd) It is necessary to calculate the amount of moles in each solution:
• 150.0mL of 0.10M KOH:
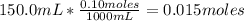
• 50.0mL of 0.20M HBr:
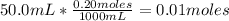
Now we know that there are 0.015 moles of KOH and 0.01 moles of HBr. Here we can see that HBr is the limiting reactant, so the 0.01 moles of HBr will react with only 0.01 moles of KOH, because the relation between the reactants is 1:1.
3rd) When the 0.01 moles of HBr is neutralized with the 0.01moles of HBr, there will still be 0.005 moles of KOH (0.015-0.01=0.005) left in the solution unneutralized.
So, we can calulate the pOH of the remaining KOH:
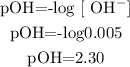
With the formula pH + pOH=14, we can calulate the pH of the final solution:
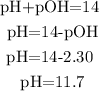
So, the pH of the solution is 11.7 (we can rounded to 12).