To solve this problem, proceed as follows: Use the molecular weight to convert the mass to moles, then, use the molarity to find the number of liters and finally convert this to mililiters (the molecular weight of CuNO3 is 125.55g/mol):
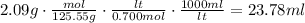
According to this, the volume of the solution is 23.78ml.