So,
The percentage yield formula is calculated to be the experimental yield divided by theoretical yield multiplied by 100.
In this question, we have that the experimental yield equals 250grams of N2, so we should find the theoretical yield using the given reaction.
First, find the moles of NaN3 that react: (To do this, we just divide the amount in grams by the molar mass of NaN3)
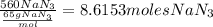
Now, let's use the reaction's stoichiometry: (For each 2 moles of NaN3, 3 moles of N2 are produced, so, we need the number of moles of N2 that can be produced from 8.6153moles of NaN3)
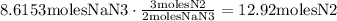
And finally, convert this amount to grams, multiplying by the molar mass of N2:
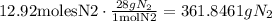
We have that the theoretical yield in the reaction was 361.8461gN2.
Now, let's replace this amount in the percentage yield formula:
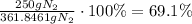
Therefore, the percentage yield was about 69.1%.