ANSWER
The initial volume of the solution is 70mL
Step-by-step explanation
Given that;
The initial concentration of the solution is 4.0%
The final concentration of the solution is 0.80%
Th e final volume of the solution of is 350mL
Follow the steps below to find the initial volume of the solution.
Step 1; Apply the dilution formula
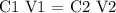

Therefore, the initial volume of the solution is 70mL