Answer:
265.9 g of glucose.
Step-by-step explanation:
What is given?
Molar enthalpy of glucose = -2538.7 kJ/mol.
Amount of energy of glucose = -3750 kJ.
Molar mass of glucose (C6H12O6) = 180 g/mol (you can calculate this using the periodic table).
Step-by-step solution:
First, we have to find the number of moles of glucose by doing a dimensional analysis of the given data. The molar enthalpy is telling us that the change of energy of 1 mol of glucose is -2538.7 kJ, so the dimensional analysis will look like this:
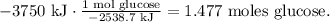
And the final step is to convert from 1.477 moles of glucose to grams using its molar mass. The conversion would be:
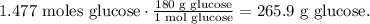
The answer is that the mass of glucose needed to produce -3750 kJ of energy is 265.9 g.