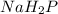The empitical formula shows the simplest ratio of elements in a compound (not the total number of atoms in the molecule).
So to find the empirical formula we need to calculate how many moles of each atom we have in this sample. Then we will see the ratio of each element.
We are given the mass, so to convert it to moles we use the molar mass. For this we go to the periodic table and see that the values for each element are:
Na (sodium): 22,99 g/mol
H (hydrogen): 1 g/mol
P (phosphorus): 25,81 g/mol
So we calculate the moles of each element as follows:
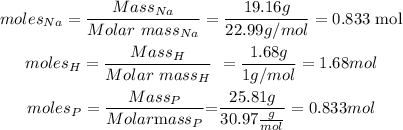
And as we see, for every 0.833 moles of Na we have the same number of moles of P, so the ratio of these elements in the molecule is 1 to 1.
As for the hydrogen:
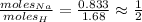
So the ratio Na to H is 1 to 2.
Now we can write the empirical formula as follows=