Answer:
The temperature of the sample is -128.14°C .
Step-by-step explanation:
1st) It is necessary to convert the1.35g of He gas to moles, using the molar mass of helium (4g/mol):
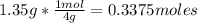
Now we know that are 0.3375moles in 1.35g of He.
2nd) With the Ideal gases formula, we ca replace the values of pressure, volume and moles to find the temperature:
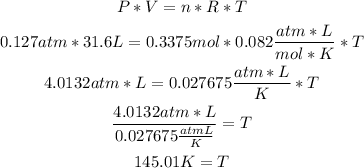
3rd) Finally, we can convert the temperature from K to °C:
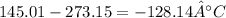
So, the temperature of the sample is -128.14°C.