Answer:
10 square units
Step-by-step explanation:
From the graph, the bounded region is a right triangle.
Base of triangle, b = 5 - 1 = 4
Altitude of triangle, h = 5 - 0 = 5
Find the area of the triangle using the formula
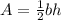
Substitute the values into the formula.
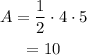
The area of the bounded region is 10 square units.