Answer:
Limiting reactant = Fe2O3.
Theoretical yield of Fe = 7.03 g.
Percent yield of Fe = 97.4 %.
Step-by-step explanation:
What is given?
Mass of Fe2O3 = 10.1 g.
Mass of CO = 13.7 g.
Molar mass of Fe2O3 = 160 g/mol.
Molar mass of CO = 28 g/mol.
Molar mass of Fe = 55.8 g/mol.
Actual experimental yield of Fe = 6.85 g.
Step-by-step solution:
To find what is the limiting reactant, we have to find the number of moles of each reactant using its respective molar mass. The conversion of 10.1 g Fe2O3 to moles is:
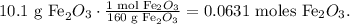
And for 13.7 g of CO:
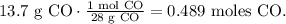
Now, let's see how many moles of Fe are being produced by each reactant. You can see in the chemical equation that 1 mol of Fe2O3 reacted produces 2 moles of Fe:
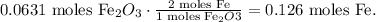
And you can see that 3 moles of CO reacted produces 2 moles of Fe:
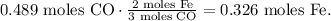
You can realize that the limiting reactant, in this case, would be Fe2O3 because this reactant is consumed first and impose the limit on the production of Fe.
Now, that we know this, we have 0.126 moles of Fe, so let's find its mass using its molar mass. This would be the theoretical yield of Fe:
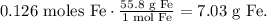
The theoretical yield of Fe is 7.03 g.
Finally, if the actual experiment yield gives us a mass of 6.85 g of Fe, and we ant to find the percent yield, we use the following formula:
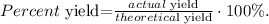
We've already known that our theoretical yield is 7.03 g and the actual yield is 6.85 g, so replacing these values in the formula we obtain:
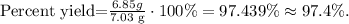
The percent yield would be 97.4 %.