Given:
The mass of the copper sample is,
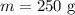
The initial temperature is,
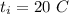
The final temperature is
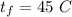
The specific heat capacity of copper is,
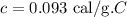
To find:
The heat generated by the electric current
Step-by-step explanation:
The heat generated by an electric current is,
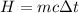
Here, the temperature difference is,
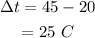
Substituting the values we get,
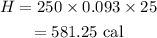
Hence, the required amount of heat is 581.25 cal.