Answer
59.5974 grams
Step-by-step explanation
Given data:
Mass of Cl2 gas, m = 23.2 g
Volume, V = 255 mL
What to find:
The mass of the gas that occupies a volume of 655 mL under the same conditions.
Solution:
The first step is to convert the mass of Cl2 to moles.
Moles = (Mass/Molar mass)
Molar mass of Cl2 gas = 71.0 g/mol
Moles = (23.2g/71.0 g/mol) = 0.3268 mol
This means 0.3268 mol Cl2 gas occupies a volume of of 255
Therefore, x mol Cl2 gas will occupy 655 mL volume.
To find x, cross multiply and divide both sides by 255 mL
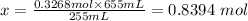
The final step is to convert the moles to mass:
Moles = (Mass/Molar mass)
0.8394 mol = Mass/71.0 g/mol
Cross multiply
Mass = 0.8394 mol x 71.0 g/mol
Mass = 59.5974 grams
Therefore, 59.5974 grams of the gas occupies a volume of 655 mL under the same conditions.