The question requires us to find the empirical formula of a sample that contains 1.3 moles of carbon, 1.3 moles of hydrogen and 13.0 moles of oxygen.
The empirical formula of a compound represents the simplest whole number ratio of atoms present in a compound. In other words, it presents the smallest whole number coefficients for each element in a molecule.
As the question mentions, the information given puts us at the last step of determining the empirical formula: we already have non-integer numbers for the coefficients of each element, and must find the integer numbers for these coefficients.
Let's suppose the coefficient of C should be 2: in this case, considering that there are 1.3 moles of C in the molecule, we would need to multiply the number 1.3 by 1.53846 to obtain the integer coefficient 2. Also, all other coefficients should be multiplied by this same number. We would have the following formula:
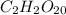
Note that these coefficients still don't represent the smallest whole numbers that we can use: we could divide all of them by 2 to obtain:
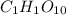
The coefficients 1, 1 and 10 for C, H and O are the smallest whole numbers we can use and they represent he simplest whole number ratio of atoms present in the compound.
As the answer requires us to put only the number that is the subscript for oxygen in the correct empirical formula, the answer should be 10.