Answer: letter A ("Subtracting the atomic number from the mass number")
Step-by-step explanation:
The question requires us to choose, among the options given, which one corresponds to the method used to determine the number of neutrons in an isotope.
Isotopes are atoms that present the same number of protons but different number of neutrons. To find the number of neutrons in an isotope, we can use the definitios of mass number and atomic number:
mass number = A = sum of protons and neutrons (A = p + n)
atomic number = Z = number of protons (Z = p)
If we rearrange the equation for mass number, we'll have:
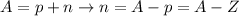
Therefore, if we subtract the atomic number of an isotope from its mass number, we'll obtain the number of neutrons.
Considering the information above, the best option to answer the question is letter A.