As per given by the question,
There are given that inequality,
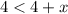
Now,
Solve the above inequality,
Then,
Substract 4 from the both side of the given inequality
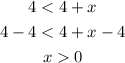
Hence, the value of given inequality will be:
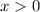
Now,
The graph of the given inequality is shown below: