Recall that:
1) The zeros of a polynomial function of the form:
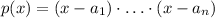
are:
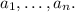
2) The set of zeros of a rational function of the form:
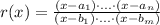
is:

Therefore the zeros of F(x) are a and b, the zeros of T(x) are c and d.
Finally, since
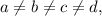
then the zeros of J(x) are a and b.
Answer: F(x) and J(x) have the same set of zeros.