We have a gas that we will assume behaves like an ideal gas, that is, there is no interaction between the gas molecules. When the temperature is constant, and what varies is the pressure and the volume, we can apply Boyle's law, which tells us:
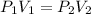
Where,
P1 is the initial pressure of the gas, 28.6atm.
V1 is the initial volume of the gas, 12.9L
P2 is the final pressure of the gas, 1.20atm
V2 is the final volume of the gas = ?
Now, we clear V2 and replace the known data:
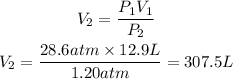
Answer: The final volume of this gas, oxygen, will be 307.5L