Step-by-step explanation
We are told that the vinegar has a pH level of 2.5
To get the hydrogen ion concentration using the properties of the Algorithm
We will substitute the values of PH =2.5 into the equation so that we will have
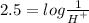
We will make
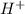
The subject of the formula
To do so, we will find the antilog of both sides
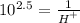
Making H the subject of the formula
Thus
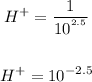
Thus, the answer is
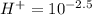
Option B is correct