To determine the mass of CaCl2 we need, we must first calculate what the molar mass of the molecule is. To find the molar mass of a molecule, we add the atomic weights of the elements multiplied by the number of atoms of each element. To see it a little clearer we can make the following table:
The molar mass of the CaCl2 molecule is 110.984 g/mol. They give us the number of moles and ask us to find the mass, we apply the following equation to find the mass of CaCl2:
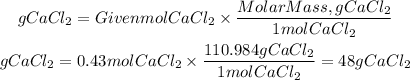
To have 0.43 moles of CaCl2 would need 48gCaCl2