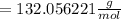We have the next chemical formula
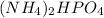
And we must determine the molar mass.
First, we must use the atomic mass of each element
- Hydrogen: 1.00794
- Nitrogen: 14.0067
- Oxygen: 15.9994
- Phosphorus: 30.973761
Then, we must replace the values of each atomical mass in the chemical formula
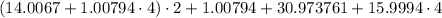
Finally, we must simplify the expression
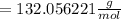
ANSWER: