Answer:
The products are: BaCO3 and LiCl, but the reaction will not occur , because BaCO3 is not soluble in water.
Step-by-step explanation:
To write the products, we have to combine the positive ion of one compound to the negative ion of the other compound.
In BaCl2, Ba+2 is the positive ion (cation) and Cl- is the negative ion (anion).
In Li2Co3, Li+1 is the cation and CO3-2 is the anion.
So, the products will be:
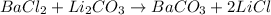
The reaction is balanced, because in each side of the reaction there are: 1 Ba, 2 Cl, 2 Li, 1C and 3 O.
Finally, BaCO3 is insoluble in water, so this reaction will not occur.