Answer:
the volume of the gas becomes 1/4 the original volume
Step-by-step explanation:
The ideal gas law gives the following relationship between temperature, pressure, and volume:
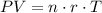
where
P = pressure
V = volume
n = number of moles
r = gas constant
T = temperature
Now let us call the initial pressure, volume, and temperature P0, V0, and T0 respectively; then we have
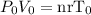
Solving for V0 gives
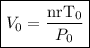
Now, what happens if the new pressure is 2 times the initial pressure ( P = 2 P0) and the new temperature is reduced to half ( T = 1/2 T0).
We find out by putting in P = 2 P0 and T = 1/2 T0 into the above equation to get:
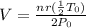
which simplifies to give us
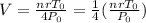
Realising that
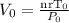
the above becomes
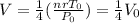
Hence, our final result is
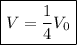
meaning that the new volume is one-fourth of the original volume.