Answer:
52 grams
Explanations:
Given the following parameters from the question:
Moles of NaOH = 1.3 moles
The formula for calculating the mass of a compound is expressed as;'
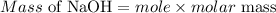
Determine the molar mass of NaOH
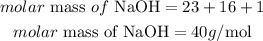
Determine the mass of NaOH
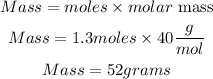
Hence the mass of NaOH present in a 1.3moles of the sample is 52 grams