Answer:
8.77 g of Ni2O3.
Step-by-step explanation:
The formation of solid nickel is given by the following reaction:
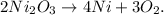
And we want to know how many grams of nickel (III) oxide (Ni2O3) we will require to form 6.25 g of Nickel (Ni). So, first, let's find the number of moles of 6.25 g of Ni using its molar mass which is 58.7 g/mol:
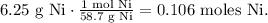
We can state a rule of three based on the chemical equation. You can see that 2 moles of Ni2O3 reacted produces 4 moles of Ni:
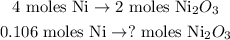
The calculation would be:
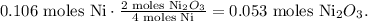
The next step is to convert 0.053 moles of Ni2O3 to grams using the molar mass of Ni2O3 which is 165.4 g/mol:
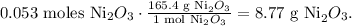
We will require 8.77 g of Ni2O3 to form 6.25 g of solid nickel.