When the Equilibrium equation for ammonia is solved, x is obtained:
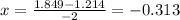
using this value of x to calculate the concentration of the other components in the equilibrium:
[H2] = 0.275mol/L
[N2]=0.901mol/L
Then we can calculate the equilibrium constant using the following equation:
![\begin{gathered} k=([H_2]^3[N_2])/([NH_3]_^2) \\ k==(0.275^3\cdot0.901)/(1.84^2)=0.0055 \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/k34v7zdksj7cqbisxiwsd2fhe6bbcycmbq.png)