To estimate the product, first we round each number to the nearest tenth:
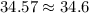
and
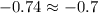
In the first number, since the second number in the decimal place of 34.57 is 7, we round up to 34.6.
In the second number -0.74, since the second number is 4 we round down to -0.7. The general rule is that if the second decimal number is greater than 5 you round up, and if is less than 5 you round down.
Now we calculate the product:
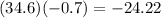
Answer: -24.22