Given:
Pressure, P = 300 kPa
Volume, V = 100 cm³
Temperature, T = 30°C
Let's fin the pressure of the gas inside the container if it is heated to 100°C.
Apply the Gay-Lussac's law:
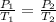
Where:
P1 = 300 kPa
T1 = 30 + 273 = 303 K
T2 = 100 + 273 = 373 K
V1 = V2 (since the container is sealed).
Let's solve for P2.
Rewrite the formula for P2:
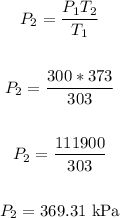
Therefore, the pressure if the container is heated to 100°C is 369.31 kPa.
ANSWER:
369.31 kPa