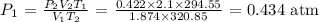Answer:
Step-by-step explanation:
Here, using the combined gas laws, we want to get the value of the initial pressure
We start by writing the combined gas law formula as follows:
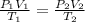
We have the terms with subscript 1 as initial values while the terms with subscript 2 are final values
Now, let us write out the given values. Kindly understand that the temperature in degrees celsius will be converted to Kelvin by adding 273.15 k to the celsius temperature
We proceed as follows:
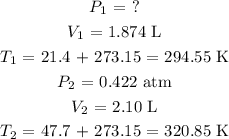
Now, we can rewrite the formula in terms of the missing value before proceeding to substitute as follows: