ANSWER
The oxidation number of chlorine in KClO3 is +5
Step-by-step explanation
Category --------- General Chemistry
Sub-category ------- Electrochemistry
The given compound is KClO3
Follow the steps below to find the oxidation number of chlorine
Let x represents the oxidation number of chlorine
Recall, the oxidation number of chlorine is +1 and the oxidation number of oxygen is -2
Hence, we have
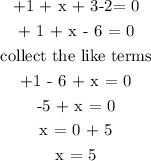
Therefore, the oxidation number of chlorine in KClO3 is +5