ANSWER
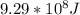
Step-by-step explanation
First, let us find the total mass of the blocks of ice:
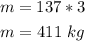
To find the amount of heat that must be gained, apply the formula for the heat change due to vaporization:
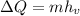
where hv = heat of vaporization = 2.26 * 10⁶ J/kg
Therefore, the amount of heat that must be gained is:
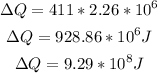
That is the answer.