Answer
260.535 grams
Step-by-step explanation
Given:
Number of moles of LiBr = 3 moles
What to find:
The grams of LiBr in 3.0 moles of LiBr
Step-by-step solution:
The molar mass of LiBr from the periodic table = 86.845 g/mol.
You can now use the mole formula to determine the grams of LiBr in 3.0 moles LiBr.
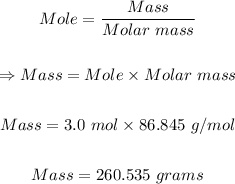
3 moles of LiBr to grams is 260.535 grams LiBr