First we need to translate the name to the compound.
trioxonitrate means we have 3 oxygens and one nigrogen.
The V means the N is 5+. If each O is 2-, we have a total of 5+ from the N and 6- from the 3 O, so the trioxonitrate(V) has a charge of 1-. Sicne it is acid, the charge is neutralized by H⁺, and since the charge is only 1-, we only need 1 H⁺.
So, the compound is HNO₃. This compound is a strong acid, which means that pratically all of the compounds dissociate in aqueous solution.

If we have a solution of 0.05 mol/dm³ and each dissociates, we will have the same concentration of H⁺:
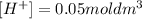
And since dm³ is the same as L, we have:
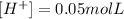
The pH can be calculated as:
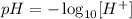
Where [H⁺] is in mol/L, so:
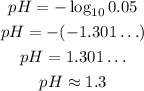
So, the pH is approximately 1.3.