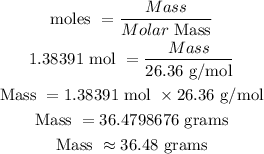Answer
36.48 grams
Step-by-step explanation:
Molarity = 1.1 M
Volume = 1258.1 mL = 1.2581 L
Molar mass = 26.36 g/mol
Firstly you need to know the number of moles of the compound.
The number of mole of the compound is calculated as follows:
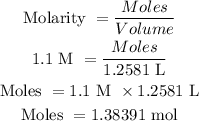
The grams of this compound would be: