Answer:
24.436L
Explanations:
According to the ideal gas equation:

where:
P is the pressure = 1atm
V is the volume
n is the number of moles
R is the gas constant
T is the temperature (in kelvin)
Given the following parameters
P = 1 atm
T = 25° or 298 K
n = 1 mole
R = 0.082 atmLmol-1K-1
Required parameter
The volume of the gas V
Substitute the given parameters into the formula to get the volume
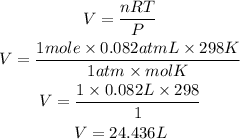
Hence the one mole of any gas occupies a volume of 24.436L