ANSWER
The initial temperature of water is 7 degrees Celcius
Option B
Step-by-step explanation
Given that;
The mass of water is 70.0 grams
The heat removed is -900J
The final temperature of the water is 4 degrees Celcius
The specific heat capacity of water is 4.182 J/g degrees celcius
To find the initial temperature, follow the steps below
Step 1; Write the formula for calculating heat energy
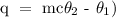
Where
q is the heat
m is the mass of the water
c is the specific heat capacity
theta 1 is the initial temperature
theta 2 is the final temperature
Step 2; Substitute the above given data into the formula in step 1
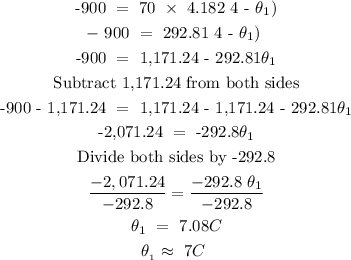
Hence, the initial temperature of water is 7 degrees Celcius