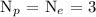ANSWER
Step-by-step explanation
Lithium is a group 1 element with an atomic number of 3
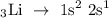
At the neutral state, the number of electrons of an atom is equal to the number of protons of an atom.
Since the atomic number is 3, therefore, the number of protons will be 3
recall, the number of protons is equal to the number of electrons
Hence, the number of electrons is 3