27200 grams
Step-by-step explanation
the density of an object is given by:
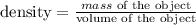
so, we have
Step 1
Let
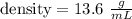
we are told that the volume is 2 Liters, so we need to convert form liters to mililiters:To convert from a larger unit to a smaller one, multiply. To convert from a smaller unit to a larger one, divide.
therefore
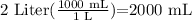
hence
volume=1000 ML
mass= unknow value= m
now, replace in the formula
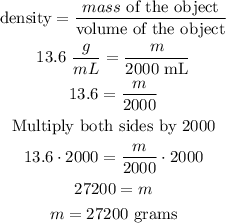
hence, the answer is 27200 grams
x