ANSWER
The mass of AlCl3 is 1250.86 grams
The mass of LiH is 298.30785 grams
Step-by-step explanation
Given reaction
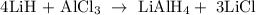
The mass of LiAlH4 is 356 grams
To find the mass of the reactants, follow the steps below
Step 1: Find the number of moles of LiAlH4 using the below formula
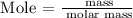
Recall, that the molar mass of LiAlH4 is 37.95 g/mol as provided in the periodic table
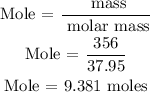
Hence, the number of moles of LiAlH4 is 9.381 moles
Step 2: Find the number of moles of LiH and AlCl3 using a stoichiometry ratio
From the reaction, 4 moles of LiH give 1 mole of LiAlH4
Let x represents the number of moles of LiH
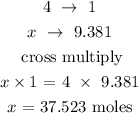
The number of moles of 37.523 moles
Find the number of moles of AlCl3?
1 mole of AlCl3 gives 1 mole LiAlH4
Let x represents the number of moles of AlCl3
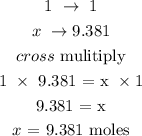
The number of moles of AlCl3 is 9.381 moles
Step 3: Find the mass of LiH and AlCl3 using the below formula
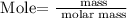
Recall, that the molar mass of LiH is 7.95 g/mol, and the molar mass of AlCl3 is 133.34 g/mol as provided in the provided table.
For LiH
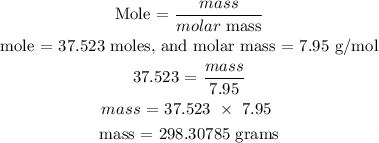
Hence, the mass of LiH is 298.30785 grams
For AlCl3
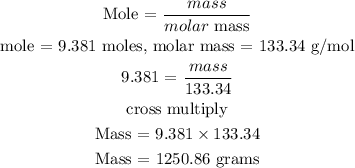
The mass of AlCl3 is 1250.86 grams