Answer:
1.053 grams of HCl.
Step-by-step explanation:
1st) It is necessary to make sure that the equation is balanced:
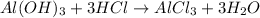
From the balanced equation we know that 1 mole of Al(OH)3 react with 3 moles of HCl.
2nd) With the stoichiometry of the reaction and the molar mass of Al(OH)3 (78g/mol) and HCl (36.5g/mol) we can calculate the grams of HCl that can react with 0.750g:
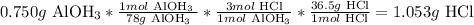
So, 1.053 grams of HCl can react with 0.750g of Al(OH)3.