the Given
*The number of mol of neon is n = 1 mol.
*The temperature of the neon gas is T = 288 K.
*The mass of the neon gas is m = 0.01 kg.
*The gas constant R is R = 8.31 J/(mol.K).
To find: The average velocity of atoms of neon (v).
According to the given equation,
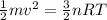
Substitute the known values.
![\begin{gathered} (1)/(2)*0.01\text{ kg}* v^2=(3)/(2)*1\text{ mol}*8.31\text{ J/mol.K}*288\text{ K} \\ 5*10^(-3)\text{ kg}* v^2=(3)/(2)*2393.28\text{ J} \\ 5*10^(-3)\text{ kg}* v^2=3589.92\text{ J}*\frac{1(kg.m^2)/(s^2)}{1\text{ J}} \\ v^2=\frac{3589.92\text{ }(kg.m^2)/(s^2)}{5*10^(-3)\text{ kg}} \\ v^2=717984(m^2)/(s^2) \\ v=\sqrt[]{717984(m^2)/(s^2)} \\ v=847.33\text{ m/s} \\ v\cong847\text{ m/s} \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/4f2miu1145c0ldxanwyy57od7jkfo5v3m2.png)
The average velocity of atoms of neon is 847.33 m/s. Thus, option (A) is the correct answer.