First, we must know what means dimensional analysis.
Dimensional analysis is the process of converting between units.
In this case we must use dimensional analysis to convert moles to atoms.
When we want to convert moles to atoms is needed the Avogadro’s number. The Avogadro's number give us that 1 mole = 6.022 x 10^23 atoms.
Then, if we need to convert moles to atoms, we must multiply the molar amount by Avogadro's number
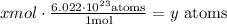
For example,
how may atoms are in six moles of sodium?
In this case since there are 6 mol of sodium, x = 6
Replacing in the formula,
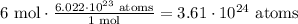
So, in six moles of sodium there are 3.61 x 10^24 atoms of sodium.
ANSWER:
if we need to convert moles to atoms, we must multiply the molar amount by Avogadro's number