Answer:
The temperature at which the reaction occurred is T = -10⁰C or 5⁰C
Step-by-step explanation:
The equation representing the number of seconds to complete a chemical reaction is:
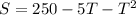
If a chemical reaction was complete in 200 seconds, then S = 200
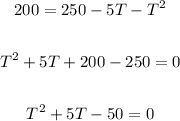
Solve for T in the quadratic equation above
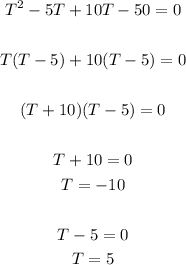
Since T = -10 or T = 5, the temperature at which the reaction occurred is T = -10⁰C or 5⁰C