a- Aluminium bromide has the following formula: AlBr₃, so the unbalanced equation is:
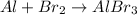
As we can see, for now the aluminium atoms are balanced, but the bromine is not. To balance the bromine, we can put 3 in front of Br₂ and 2 in front of AlBr₃. That way, we will have a total of 6 bromine atoms in each side:
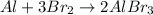
But now the Al is unbalaced, so to fix it we can add a 2 in front of Al to get the balanced equation:
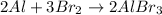
b- The aluminium are the lone atoms, so, counting them, we see that there are 8 atoms initially.
c- Each pair of empty circles represent a molecule of Br₂, counting them we have 6 molecules initially.
d- The proportion of Al to AlBr₃ is 2:2, that is, 1:1, so if all Al reacts, we would produce the same amount of AlBr₃ as Al, which would be 8 molecules.
The proportion of Br₂ to AlBr₃ is 3:2, so is all Br₂ reacts we will get 2/3 of that as AlBr₃, which would be 6*2/3 = 4 molecules.
This shows that there is not enough Br₂ to react with all 8 atoms of Al, meaning only 4 molecules of AlBr₃ will be produced.
e- Since there is not enough Br₂ to react with all Al present, the limiting reactant is the bromine.
f- The excess reactant is the other one, so if bromine is the limiting, the aluminium is the excess reactant.
g- Since only 4 molecules of AlBr₃ will be formed with all the bromine present, since the proportion of Al to AlBr₃ is 1:1, we wil need only 4 atoms of Al to produce them, which meand that, from the total 8 atoms, we will get
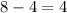
4 atoms of Al as excess reactant after the reaction is complete.