Answer:
0.492 moles
Explanations:
Given the chemical equation between phosphorous and oxygen as shown:
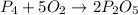
Given the following parameters
Moles of oxygen = 1.23 moles of O2
According to stoichiometry, 5moles of oxygen produces 2 moles of diphosphorus pentoxide, the moles of diphosphorus pentoxide required is given as:
Moles of P2O5 = 2/5 * 1.23
Moles of P2O5 = 0.492moles
Hence the required moles of diphosphorus pentoxide that can be made is 0.492 moles