Step-by-step explanation:
Given:
T₁ = 273 K
P₁ = 0.75 atm
T₂ = 350 K
What to find:
The final pressure, P₂
According to Gay-Lussac's law which states that the pressure exerted by a given amount of gas kept at a constant volume is directly proportional to the absolute temperature of the gas. Mathematically,
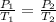
Using the formula above, substitute the given parameters to find P₂:
![undefined]()