The concentration is measured by molarity, the formula of molarity is:
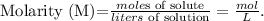
Based on the given data, we've already had the volume in liters and we have to convert 6.25 grams of HCl to moles. We have to use the molar mass of HCl which is 36.4 g/mol (you can find the molar mass using the periodic table). The conversion would be:
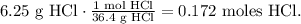
And the final step is to replace the values we have in the formula of molarity:
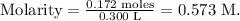
The molarity of 6.25 g of HCl in 0.300 L of solution is 0.573 M.