Answer: the molarity of the H2SO4 solution is 0.28 M
Step-by-step explanation:
The question requires us to calculate the molarity of a sulfuric acid (H2SO4) solution, knowing that it is necessary 19.7 mL of a 0.72 M sodium hydroxide (NaOH) solution to neutralize 25.0 mL of the acid solution.
To solve this problem, we'll need to consider the chemical reaction between H2SO4 and NaOH:
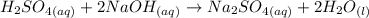
Note that the chemical equation above is already balanced and it tells us that 2 moles of NaOH are necessary to completely react (in other words, to completely neutralize) 1 mol of H2SO4.
Therefore, at the end point of the titulation (where the neutralization occurs), we would need:
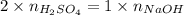
where n corresponds to the number of moles of a substance.
Knowing that the molarity of a solution (C, in mol/L or M) is defined as the number of moles (n) divided by the volume of the solution (V, in liters), we can write the number of moles as the product between C and V:
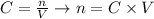
And we can rewrite the equation for the end point of the reaction as:
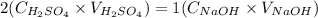
We can rearrange the equation above to calculate the molarity of H2SO4:
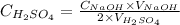
The question provided the following values:
C(H2SO4) = ? (needs to be calculated)
V(H2SO4) = 25.0mL
C(NaOH) = 0.72 M
V(NaOH) = 19.7mL
Replacing these values to calculate the molarity of H2SO4, we'll have:
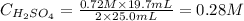
Therefore, the molarity of the H2SO4 solution is 0.28 M.